How was the electronegative values of the carbon atom crossed by sp3 hybridization calculated as 2.48, in sp2 hybridization equal to 2.6, and in sp hybridization equals 2.99? - Quora
![SOLVED: NFz is polar while BFz is nonpolar Explain this difference, using drawings and words. [6 points] Atom Electronegativity Value Boron Bromino 2.8 Carbon 2,5 Fluorine 4,0 Hydrogen 2,1 Nitrogen Oxygen 3,0 3,5 SOLVED: NFz is polar while BFz is nonpolar Explain this difference, using drawings and words. [6 points] Atom Electronegativity Value Boron Bromino 2.8 Carbon 2,5 Fluorine 4,0 Hydrogen 2,1 Nitrogen Oxygen 3,0 3,5](https://cdn.numerade.com/ask_images/050d72c8cacb45e9b16d8090ae90fead.jpg)
SOLVED: NFz is polar while BFz is nonpolar Explain this difference, using drawings and words. [6 points] Atom Electronegativity Value Boron Bromino 2.8 Carbon 2,5 Fluorine 4,0 Hydrogen 2,1 Nitrogen Oxygen 3,0 3,5
Electronegativity (a) and hardness (b) versus field strength for carbon... | Download Scientific Diagram
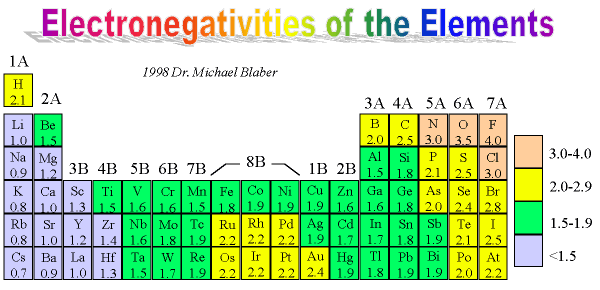
Where can I find electronegativities of lithium, carbon, and fluorine? How do they relate to the tendency to obtain a noble gas configuration? | Socratic
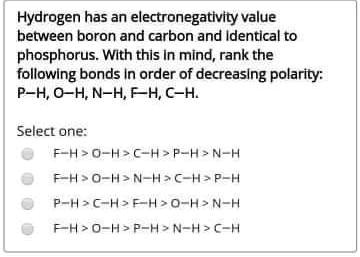
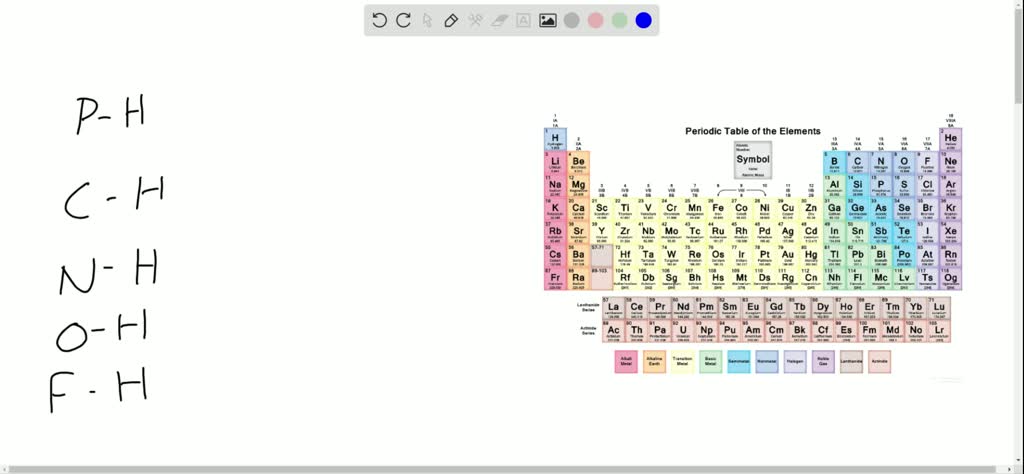
SOLVED: Hydrogen has an electronegativity value between boron and carbon and Identical [0 phosphorus. With this In mind, rank the following bonds In order of decreasing polarity: P-H,0-HN-HFHC-H Select one: F-H>o-H>C-H>P-H>N-H Fh>0-A>N-H>C-A>P-H

Electronegativity, Basic Introduction, Periodic Trends - Which Element Is More Electronegative? - YouTube

Carbon Chemical Element First Ionization Energy Stock Vector (Royalty Free) 1193986345 | Shutterstock

The role of the electronegativity in the charge of the carbon atom. V... | Download Scientific Diagram
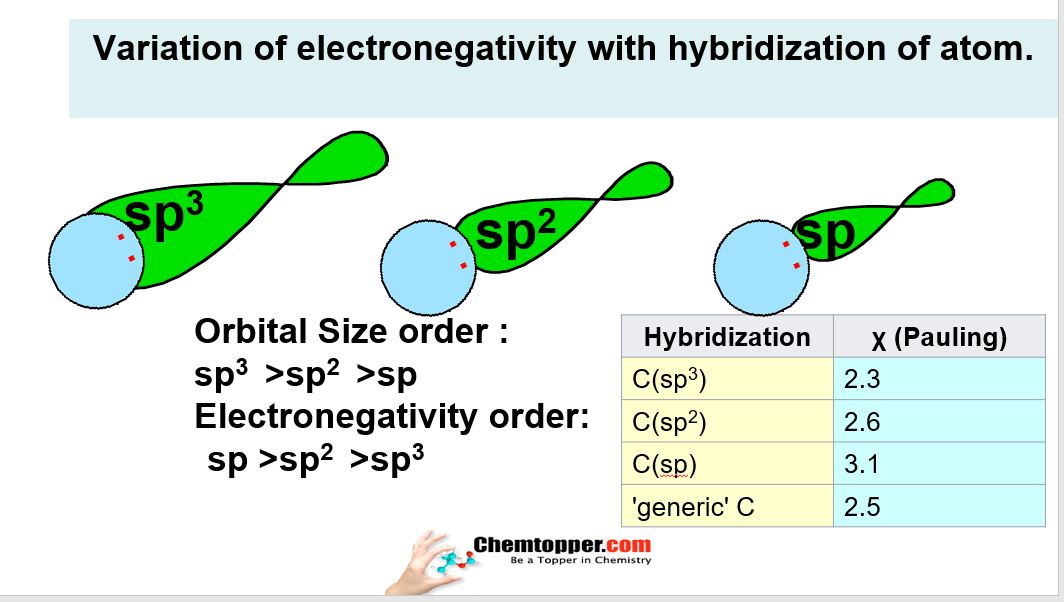
Electronegativity Part 4 – Polarity of bonds in organic molecules based on hybridization ,oxidation number and formal charges. – Online Chemistry Tutor

Question Video: Determining the Polarity of the Bond and Overall Molecule of Molecular Nitrogen | Nagwa
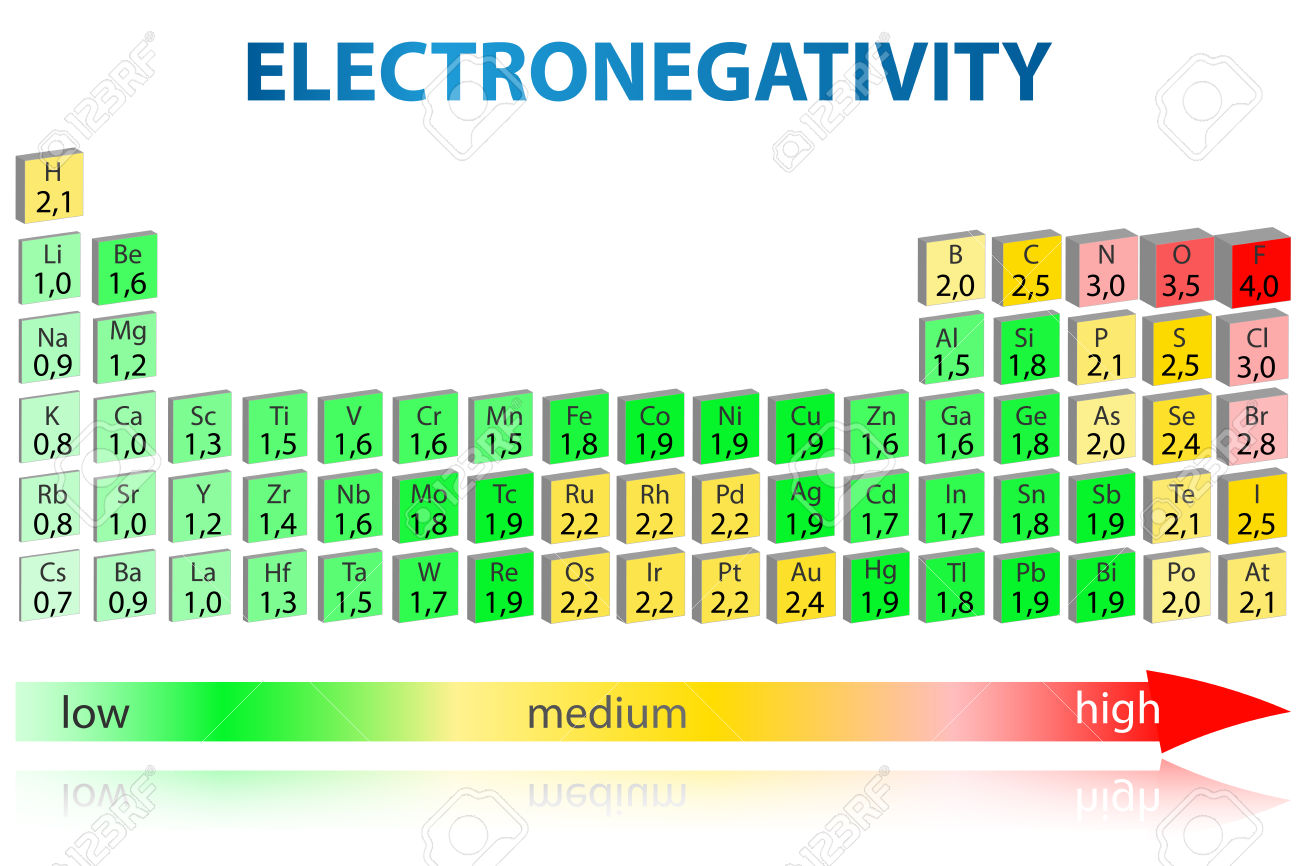
Which group of elements is listed in order of increasing electronegativity? a) F, Cl, Ge, Sn b) Rb, Ca, Sc, Cs c) Zr, V, Nb, Ta d) Sn, As, P, S e)