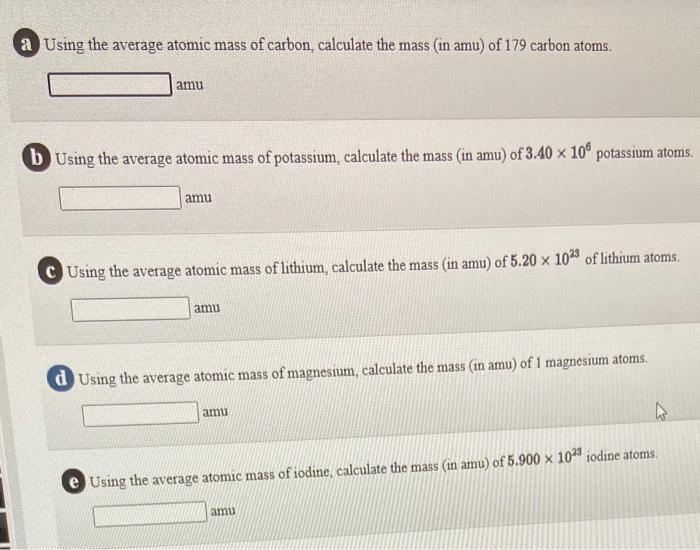
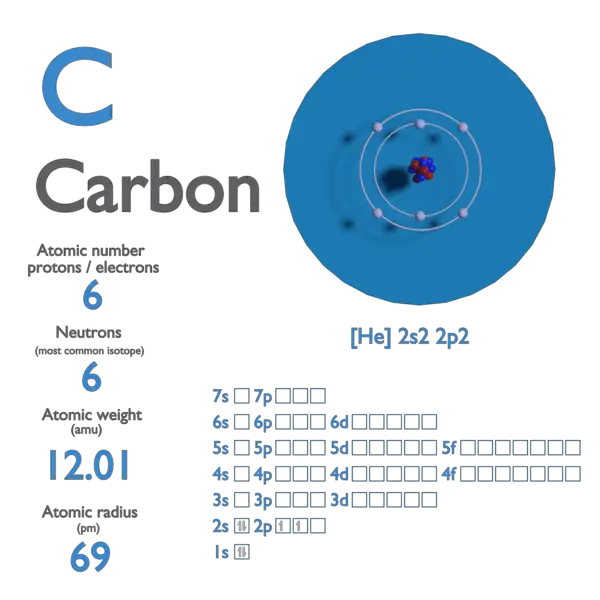
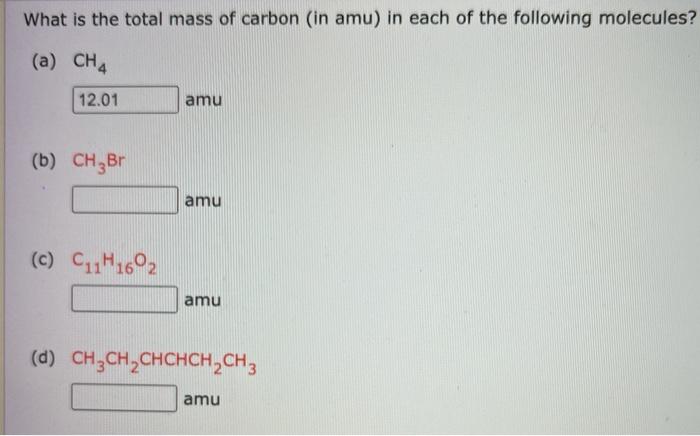
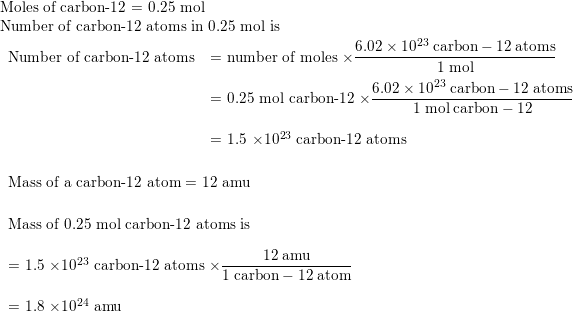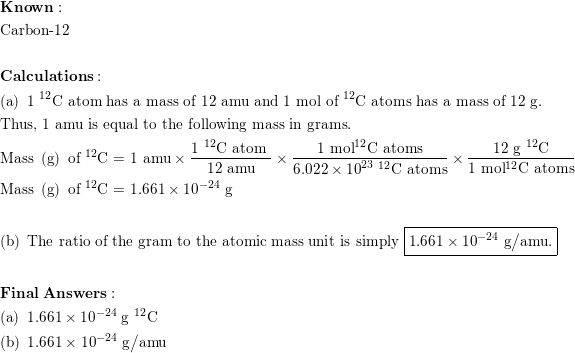1: a) what is the mass in amu of a carbon 12 atom? b) why is the atomic weight of carbon reported as 12.011 in the table of elements and the periodic

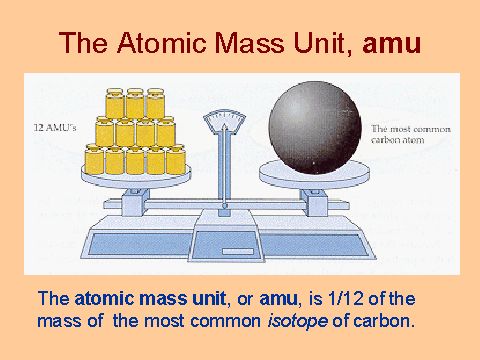
Atomic Mass Standard mass unit is derived from carbon 12 Atomic mass unit – the mass equal to 1/12 the mass of one Carbon 12 atom. - ppt download

If the atomic weight of carbon is set at 24 amu, the value of the avogadro constant would be :- - YouTube
1. If we assume 1/24th part of mass of carbon instead of 1/12th part of it as 1 amu , then mass of 1 mole of a substance will :A) remain changedB)

SOLVED: (a) What is the mass in amu of a carbon-12 atom? (b) Why is the atomic weight of carbon reported as 12.011 in the table of elements and the periodic table

SOLVED: 12.011 amu is the average atomic mass of a carbon atom. Imagine that you are able to pick up only one carbon atom from a sample, what are the chances that